Burkitt’s lymphoma is a rare but highly aggressive non-Hodgkin B-cell lymphoma, and an intensive systemic chemotherapy is the standard treatment of choice, with major drawbacks for the patient. The scientific research is now moving towards the design of targeted therapeutic tools able to be completely safe per se, and simultaneously be capable of becoming selectively toxic when externally activated by stimuli, to reduce therapeutic side effects.
This case of study focuses its attention on the therapeutic efficacy of an innovative, groundbreaking and combined therapy based on administration of targeted biomimetic zinc oxide nanocrystals
(ZnO NCs) and ultrasounds. These engineered nanocrystals are safe by design, but when exposed to ultrasound they become significantly toxic for cells in which they are internalized. A customized biomimicking lipidic shield, resembling the composition of natural extracellular vesicles, entraps ZnO NCs, and allows the internalization of these nanotools preferentially into Burkitt’s lymphoma cells due to targeting antibody fragments encased in the lipidic shield.
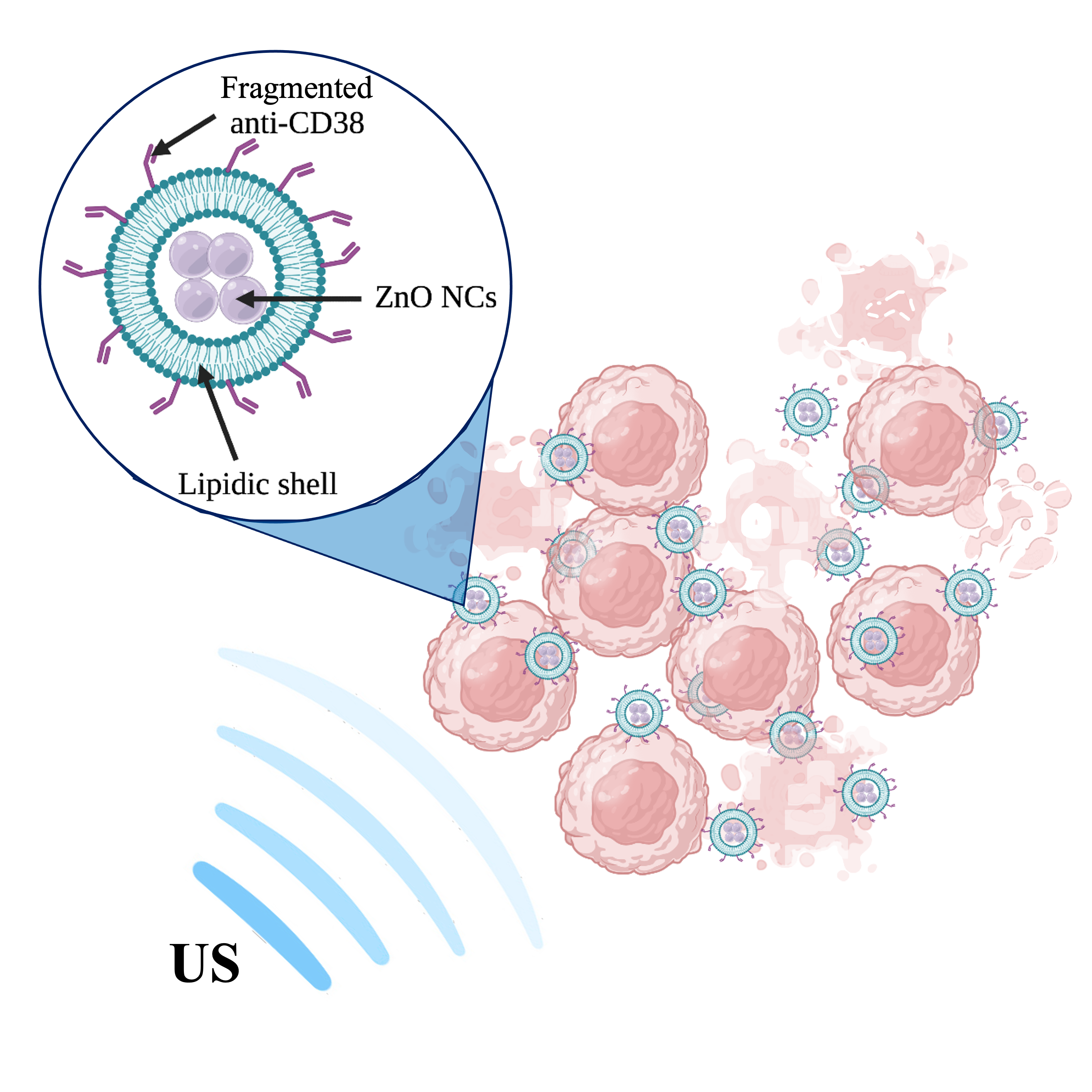
Their cytotoxicity capability is externally enabled by the US irradiation. The synergistic interaction between US and targeted hybrid NPs leads to an in vitro significant killing efficacy. The cell viability of healthy B-lymphocytes and a targeting-negative control cancer cell line is not affected by the combined treatment, underling the highly specific and targeted behaviour of the proposed therapeutic nanoagent. This work deeply characterizes the hybrid targeted nanocontructs and narrow its focus to the biological effects resulting from interaction of hybrid NCs and ultrasound. Cytoflurimetric analysis, fluorescent microscopy and various biological assay were exploited to verify the effectiveness of the proposed pioneering therapy for Burkitt’s lymphoma.
See our publications on this topic:
